Exergonic Vs Exothermic
In the fascinating world of chemistry, understanding the difference between exergonic and exothermic processes is essential. These terms describe fundamental concepts that help us comprehend the energy changes that occur during chemical reactions. In this comprehensive article, we will delve deep into the realm of exergonic and exothermic processes, exploring their definitions, characteristics, and real-world applications.
Unveiling the Essence of Exergonic and Exothermic Processes

Chemical reactions are at the heart of countless natural phenomena and technological advancements. Two key terms, exergonic and exothermic, are often used to describe the energy transformations that take place during these reactions. While they might sound similar, they represent distinct aspects of energy flow and provide valuable insights into the behavior of chemical systems.
Exergonic Processes: Unleashing Energy Potential
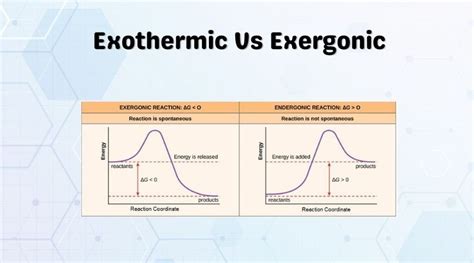
An exergonic process is a reaction that releases energy to its surroundings. In simpler terms, it is a process where the system loses energy, often in the form of heat or work. This release of energy is associated with a decrease in the system’s overall energy content. Exergonic reactions are spontaneous and occur without the need for external energy input.
One of the most well-known examples of an exergonic process is cellular respiration, the biochemical pathway by which living organisms convert nutrients into energy. During cellular respiration, glucose molecules react with oxygen, resulting in the release of energy in the form of adenosine triphosphate (ATP). This energy-rich molecule powers various cellular processes, enabling organisms to perform essential functions.
Exergonic reactions are not limited to biological systems. Consider the combustion of fuels, such as gasoline in an internal combustion engine. When gasoline burns, it undergoes an exergonic reaction, releasing a substantial amount of energy in the form of heat and light. This energy is harnessed to propel vehicles and power various mechanical systems.
The concept of exergonicity is crucial in understanding the thermodynamics of chemical reactions. According to the Second Law of Thermodynamics, exergonic processes tend to occur spontaneously because they lead to an increase in the entropy (disorder) of the universe. This law underpins our understanding of why certain reactions proceed without intervention and are essential for maintaining equilibrium in natural systems.
Exothermic Reactions: Unveiling Heat Release
On the other hand, an exothermic reaction is characterized by the release of heat to the surroundings. In such reactions, the system’s internal energy decreases, and this excess energy is transferred to the environment as thermal energy. Exothermic reactions are often accompanied by a rise in temperature, making them readily observable.
One classic example of an exothermic reaction is the combustion of natural gas for heating purposes. When natural gas (mostly methane) is ignited, it undergoes a rapid exothermic reaction, releasing a significant amount of heat. This heat is utilized to warm homes, industrial facilities, and even provide energy for cooking.
Another familiar exothermic process is the combustion of fuels in internal combustion engines, as mentioned earlier. The energy released during combustion is converted into mechanical work, propelling vehicles and machinery. The heat generated also plays a vital role in maintaining the engine’s optimal operating temperature.
Exothermic reactions are not limited to combustion processes. In chemical synthesis, the formation of new compounds often involves exothermic reactions. For instance, the synthesis of ammonia from nitrogen and hydrogen gas is highly exothermic, releasing a substantial amount of heat energy.
| Process | Energy Flow | Real-World Example |
|---|---|---|
| Exergonic | Releases energy to surroundings | Cellular respiration |
| Exothermic | Releases heat to surroundings | Combustion of natural gas |

Analyzing the Energy Landscape: Key Considerations
When studying exergonic and exothermic processes, several critical factors come into play. These considerations help us understand the intricate dynamics of energy transformations and their implications.
Activation Energy and Reaction Rates
One crucial aspect is the concept of activation energy. Every chemical reaction has an energy barrier that must be overcome for the reaction to proceed. This energy barrier is known as the activation energy. In exergonic reactions, the activation energy required is often lower compared to endothermic (energy-absorbing) reactions. This lower energy barrier contributes to the spontaneity of exergonic processes.
The rate of reaction is another vital consideration. Exergonic reactions, due to their inherent energy-releasing nature, often exhibit higher reaction rates compared to endothermic reactions. This is because the release of energy provides the necessary activation energy for subsequent reaction steps, facilitating a more rapid progression of the reaction.
Thermodynamic Favorability and Equilibrium
The thermodynamic favorability of a reaction is determined by its Gibbs free energy change (ΔG). Exergonic reactions are characterized by a negative ΔG, indicating that the reaction is energetically favorable and will proceed spontaneously. In contrast, endothermic reactions have a positive ΔG, requiring an input of energy to proceed.
Understanding the equilibrium of reactions is essential. While exergonic reactions tend to drive the system towards completion, they may not necessarily reach equilibrium. The extent to which a reaction progresses depends on various factors, including the reaction conditions, the presence of catalysts, and the overall thermodynamic stability of the products.
Energy Conversion and Efficiency
In practical applications, the efficiency of energy conversion is a critical consideration. Exergonic processes, especially those involving the conversion of chemical energy into mechanical work or electricity, must be optimized to maximize energy efficiency. This involves minimizing energy losses and ensuring that the released energy is harnessed effectively.
For instance, in renewable energy systems like solar panels or wind turbines, the exergonic nature of sunlight absorption or wind-driven motion is utilized to generate electrical energy. Engineers and scientists work tirelessly to enhance the efficiency of these systems, ensuring that a maximum amount of energy is captured and converted into usable power.
Applications and Real-World Impact
The understanding of exergonic and exothermic processes has far-reaching implications across various scientific and technological fields.
Biochemistry and Life Processes
In the realm of biochemistry, the concept of exergonic reactions is fundamental to understanding life itself. From the aforementioned cellular respiration to the intricate processes of DNA replication and protein synthesis, exergonic reactions provide the energy currency required for life to flourish.
Biochemists and molecular biologists study these energy-releasing reactions to uncover the intricate mechanisms that govern cellular function. This knowledge has led to breakthroughs in medicine, such as the development of targeted therapies and a deeper understanding of metabolic disorders.
Chemical Synthesis and Industrial Processes
Exothermic reactions are at the heart of many chemical synthesis processes in the industrial sector. The controlled release of heat energy is harnessed to drive reactions and produce a vast array of chemicals and materials.
For instance, in the petrochemical industry, exothermic reactions are employed to crack complex hydrocarbon molecules into simpler, more useful compounds. This process is essential for the production of fuels, lubricants, and various chemical feedstocks.
Additionally, the understanding of exothermic reactions is crucial for process safety in chemical plants. Engineers and safety experts work together to design and operate processes that effectively manage the heat generated by exothermic reactions, preventing accidents and ensuring the well-being of workers and the environment.
Environmental Considerations
The study of exergonic and exothermic processes also has significant implications for the environment. Exergonic reactions, such as those in cellular respiration, are fundamental to the carbon cycle and the overall energy balance of ecosystems.
Moreover, the efficient utilization of exergonic and exothermic reactions can contribute to sustainable energy practices. For example, the optimization of combustion processes in power plants can lead to reduced greenhouse gas emissions and improved energy efficiency.
Conclusion: Unlocking the Power of Energy Transformations
In this comprehensive exploration, we have unraveled the intricacies of exergonic and exothermic processes, shedding light on their distinct characteristics and real-world applications. From the energy-releasing potential of exergonic reactions to the heat-generating nature of exothermic processes, these concepts underpin our understanding of chemical reactions and their impact on various scientific and technological domains.
By delving into the energy landscape of chemical reactions, we gain insights into the fundamental principles that govern our world. Whether it’s the spontaneous energy release in biological systems or the controlled harnessing of heat in industrial processes, the study of exergonic and exothermic reactions continues to shape our understanding of the universe and drive innovations that improve our lives.
What is the difference between exergonic and exothermic reactions in terms of energy release?
+Exergonic reactions release energy to their surroundings, often in the form of heat or work. Exothermic reactions specifically release heat to the environment. While both involve energy release, exergonic reactions are more general, encompassing various forms of energy transfer, while exothermic reactions are focused on thermal energy release.
Are all exergonic reactions exothermic, and vice versa?
+No, not all exergonic reactions are exothermic, and not all exothermic reactions are exergonic. Exergonic reactions are defined by the release of energy, which can be in various forms, including heat. Exothermic reactions, on the other hand, specifically involve the release of heat. Therefore, an exergonic reaction can be exothermic if heat is the primary form of energy release, but it can also be non-exothermic if the energy is released in other forms.
How do exergonic reactions relate to the Second Law of Thermodynamics?
+The Second Law of Thermodynamics states that in any energy exchange, the total entropy (disorder) of the universe increases. Exergonic reactions are spontaneous processes that occur without external energy input and often lead to an increase in entropy. These reactions are energetically favorable and contribute to the overall increase in entropy, aligning with the principles of the Second Law.