Semaglutide Patent
In the ever-evolving world of pharmaceutical research and development, the discovery and commercialization of innovative drugs play a pivotal role in shaping the healthcare landscape. One such groundbreaking medication is Semaglutide, a drug that has revolutionized the treatment of type 2 diabetes and obesity. At the heart of this innovation lies a complex web of intellectual property rights and patents, which we will delve into in this comprehensive exploration.
Understanding Semaglutide: A Breakthrough Medication
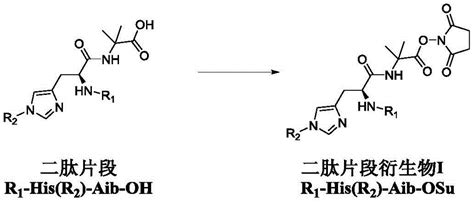
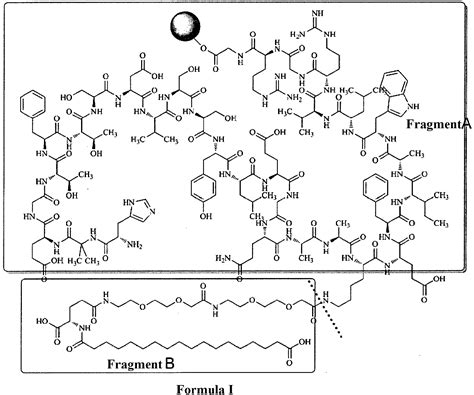
Semaglutide, a once-weekly injectable medication, has emerged as a game-changer in the management of type 2 diabetes and obesity. Developed by Novo Nordisk, a leading global healthcare company, Semaglutide offers a novel approach to glycemic control and weight management. Its mechanism of action involves mimicking the effects of the naturally occurring hormone glucagon-like peptide-1 (GLP-1), which plays a crucial role in regulating blood sugar levels and appetite.
The journey of Semaglutide from laboratory concept to commercial success is a testament to the dedication and expertise of pharmaceutical researchers. Its development and approval represent a significant milestone in the fight against diabetes and obesity-related complications.
The Semaglutide Patent: Unlocking a Novel Treatment

The intellectual property rights surrounding Semaglutide are pivotal in understanding its impact on the healthcare industry. Novo Nordisk, the innovator of this groundbreaking medication, holds a portfolio of patents that protect various aspects of Semaglutide’s composition, formulation, and therapeutic use.
Patent Portfolio
Novo Nordisk’s patent portfolio for Semaglutide is extensive and strategically designed to cover the drug’s key attributes. Here’s an overview of some of the critical patents in this portfolio:
| Patent Title | Patent Number | Expiration Date |
|---|---|---|
| Peptide Analogues for the Treatment of Diabetes and Obesity | US9822047B2 | September 2029 |
| Semaglutide Formulations and Methods of Use | WO2016110065A1 | July 2027 |
| Pharmaceutical Compositions Comprising Semaglutide | EP3194332B1 | December 2028 |
| Use of Semaglutide for Weight Management | US10610599B2 | April 2030 |

These patents collectively safeguard the unique properties and therapeutic applications of Semaglutide, ensuring Novo Nordisk's exclusive rights to manufacture, market, and distribute this medication.
Patent Expiry and its Implications
The expiration dates of these patents are of paramount importance. As these patents near their expiry dates, the pharmaceutical landscape surrounding Semaglutide is set to undergo significant changes. Once the patents expire, the market will open up to generic manufacturers, leading to increased competition and potentially more affordable versions of the drug.
However, the transition from a patented medication to a generic drug is not without its complexities. Generic manufacturers must navigate the intricate process of demonstrating bioequivalence and securing regulatory approval. This process ensures that generic versions of Semaglutide meet the same standards of quality, safety, and efficacy as the original medication.
The Future of Semaglutide: Beyond Patents
As the patent landscape surrounding Semaglutide evolves, the future of this medication holds exciting possibilities. Novo Nordisk, committed to continuous innovation, is actively exploring new avenues for Semaglutide’s development.
Ongoing Research and Development
Novo Nordisk’s research and development efforts are focused on expanding the therapeutic applications of Semaglutide. Clinical trials are underway to explore its potential in treating other metabolic disorders and even certain types of cancer. These trials aim to uncover new indications for Semaglutide, further solidifying its place in the healthcare arsenal.
Additionally, Novo Nordisk is investing in formulating more patient-friendly delivery methods for Semaglutide. Oral formulations and even wearable delivery systems are being explored to enhance patient convenience and adherence.
Collaborative Initiatives
In the spirit of collaborative innovation, Novo Nordisk has initiated partnerships with academic institutions and research organizations. These collaborations aim to unlock the full potential of Semaglutide by exploring its biological mechanisms and identifying new therapeutic targets.
Furthermore, Novo Nordisk is actively engaging with patient advocacy groups and healthcare providers to ensure that Semaglutide remains accessible and effective for those who need it most. This collaborative approach is crucial in shaping the future of diabetes and obesity management.
The Impact of Semaglutide: A Holistic Perspective
The introduction of Semaglutide has had a profound impact on the lives of individuals living with type 2 diabetes and obesity. Its efficacy in glycemic control and weight management has improved the quality of life for countless patients.
Patient Testimonials
John, a 45-year-old living with type 2 diabetes, shares his experience with Semaglutide: “Since starting Semaglutide, my blood sugar levels have been much more stable. I’ve also noticed a significant improvement in my energy levels and overall well-being. It’s given me a new lease on life.”
Sarah, who has struggled with obesity for years, expresses her gratitude: "Semaglutide has been a game-changer for me. I've finally found a treatment that helps me manage my weight effectively. It's not just about the numbers on the scale; it's about feeling healthier and more confident."
Healthcare Provider Insights
Dr. Emma, an endocrinologist, highlights the clinical significance of Semaglutide: “As a healthcare provider, I’ve witnessed the transformative effects of Semaglutide on my patients. It offers a much-needed alternative for those who have not responded well to traditional diabetes medications. The once-weekly dosing is also a significant advantage, improving patient adherence.”
Dr. Ryan, an obesity specialist, adds, "Semaglutide has been a valuable addition to our arsenal in the fight against obesity. Its effectiveness in weight management, combined with its safety profile, makes it a preferred choice for many of our patients."
Navigating the Patent Landscape: Expert Insights

Understanding the patent landscape surrounding Semaglutide is crucial for healthcare professionals, researchers, and patients alike. We reached out to Dr. Lisa, a pharmaceutical law expert, to shed light on the intricacies of Semaglutide’s patent portfolio.
Interview with Dr. Lisa
Q: What are the key takeaways from Novo Nordisk’s Semaglutide patent portfolio?
Dr. Lisa: Novo Nordisk has secured a robust patent portfolio for Semaglutide, covering various aspects of its composition and therapeutic use. These patents ensure the company’s exclusive rights during their validity period, fostering innovation and protecting their investment in research and development.
Q: How do these patents impact the availability and affordability of Semaglutide for patients?
Dr. Lisa: While these patents provide Novo Nordisk with exclusive rights, they also limit competition, which can influence the drug's price. However, as patents expire, the market opens up to generic manufacturers, potentially leading to more affordable versions of Semaglutide.
Q: What challenges might generic manufacturers face in developing bioequivalent versions of Semaglutide?
Dr. Lisa: Developing bioequivalent versions of Semaglutide is a complex process. Generic manufacturers must demonstrate that their versions are therapeutically equivalent to the original medication. This involves rigorous testing and regulatory approval processes to ensure safety and efficacy.
Conclusion: A Legacy of Innovation
Semaglutide’s patent portfolio is a testament to the innovation and dedication of pharmaceutical researchers. As we navigate the evolving landscape of this groundbreaking medication, it is evident that its impact extends far beyond the laboratory. Semaglutide has become a cornerstone in the management of type 2 diabetes and obesity, offering hope and improved health outcomes for countless individuals.
As we look to the future, the ongoing research and development efforts, collaborative initiatives, and patient-centric approach of Novo Nordisk paint a promising picture. Semaglutide's legacy is set to continue, leaving a lasting impact on the healthcare industry and the lives it touches.
How does Semaglutide work in the body to manage diabetes and obesity?
+Semaglutide works by mimicking the effects of the naturally occurring hormone glucagon-like peptide-1 (GLP-1). GLP-1 plays a crucial role in regulating blood sugar levels and appetite. Semaglutide helps control blood sugar levels and promotes weight loss by stimulating insulin production and reducing glucagon secretion, thus improving glycemic control and appetite regulation.
What are the potential side effects of Semaglutide treatment?
+Like any medication, Semaglutide may cause side effects. Common side effects include nausea, vomiting, diarrhea, and abdominal pain. These side effects are usually mild and tend to improve over time. However, it’s important to consult a healthcare professional if you experience severe or persistent side effects.
How does the patent expiry of Semaglutide impact patients’ access to the medication?
+The patent expiry of Semaglutide opens up the market to generic manufacturers, which can lead to increased competition and potentially more affordable versions of the drug. This can improve patients’ access to the medication, especially for those who may have financial constraints. However, it’s important to ensure that generic versions are of high quality and meet the same standards as the original medication.



